सीएसआईआर - कोशिकीय एवं आणविक जीवविज्ञान केन्द्र
वैज्ञानिक तथा औद्योगिक अनुसंधान परिषद
भारत का नवप्रवर्तन इंजन
Date : अगस्त 29, 2024
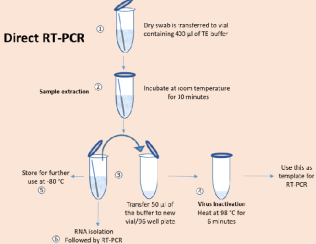
New Delhi, 26th November 2020 and fast method of Dry Swab developed by CSIRs constituent lab Centre for Cellular and Molecular Biology (CCMB) Hyderabad for scaling up of SARS detection has now been approved by ICMR based on their independent validation. This method developed by CSIRvariation of the existing gold standard RT method and can easily scale up the testing b to 3 fold with no new investment of resources. After evaluating this method and finding an overall concordance of 96.9%, ICMR has now issued an advisory for the use of CSIR swab method, considering its lesser cost and quick turn-around time.
CSIR-CCMB, Hyderabad has been testing samples for coronavirus since April 2020. Having worked closely with the healthcare workers of Telangana, it identi of the key issues that slow the testing process. In response to it, the researchers here developed the extraction free testing method for the COVID-19 virus.
More specifically, the Dry Swab PCR method involves collecting an transporting the nasal swab in dry state (as opposed to using the viral transport.
medium VTM) which makes the transportation and handling of the samples easy and less prone to spillage and spread of infection. Secondly, the step of RNA isolation from the sample is omitted and involves only simple processing of the sample followed by direct RT-PCR using the kit recommended by ICMR. Omitting the step of RNA isolation offers a huge benefit over the conventional method, as the RNA isolation is a major bottleneck in terms of time, cost and trained manpower. Given this, with the same resources and no additional cost more samples can be tested and can be easily scaled up at least 2-3 times immediately.
DG-CSIR, Dr Shekhar C Mande, commenting the development said that the Dry-Swab Direct RTPCR method is easy to implement with no requirement of new kits and existing manpower can perform this with no additional training and hence could make a significant contribution to ramping up the testing capacity in the country quickly.
Dr Rakesh Mishra, Director, CCMB adds, “RNA extraction, even with automation, takes 4 hours for roughly 500 samples. VTM and RNA extraction both add a significant burden on money and time required for mass testing for coronavirus. We believe the technique’s merit holds for all kinds of settings and has the potential of bringing the costs and time of testing by 40-50%”.
Significantly, the modified method of CSIR-CCMB has also been independently corroborated by multiple premier institutes and hospitals such as Centre for DNA Fingerprinting and Diagnostics (CDFD), IISER-Berhapmur, CSIR-NEERI, GMCH-Nagpur, Genepath based in Pune, IGGMSH and MAFSU, Nagpur and also Apollo Hospitals, Hyderabad. Further, this modified method has been published in peer reviewed journal by CSIR-CCMB and by other scientific groups in several prestigious scientific journals across the world.
 Advertisement no 07/10 for the post Junior Scientist.
Advertisement no 07/10 for the post Junior Scientist.
 List of shortlisted candidates for the temporary positions against CCMB Web Notif.No.0724/B- [26-08-2024]
List of shortlisted candidates for the temporary positions against CCMB Web Notif.No.0724/B- [26-08-2024]
 Result of selected candidates for the temporary positions against CCMB Web Notif.No.0724/A - [21-08-2024]
Result of selected candidates for the temporary positions against CCMB Web Notif.No.0724/A - [21-08-2024]
 Notification of Schedule for Trade Test and Downloading of Admit Cards for the posts of Gr. II (1)/Technician (1) against Advt.No:01/2021 - [19-08-2024]
Notification of Schedule for Trade Test and Downloading of Admit Cards for the posts of Gr. II (1)/Technician (1) against Advt.No:01/2021 - [19-08-2024]
 List of selected candidates for the temporary positions against CCMB Web Notif.No.0624/A - [14-08-2024]
List of selected candidates for the temporary positions against CCMB Web Notif.No.0624/A - [14-08-2024]
 List of shortlisted candidates for the temporary positions against CCMB Web Notif.No.0724/A - [02-08-2024]
List of shortlisted candidates for the temporary positions against CCMB Web Notif.No.0724/A - [02-08-2024]
 Notification No.0824/A for various temporary positions on contractual basis- [02-08-2024]
Notification No.0824/A for various temporary positions on contractual basis- [02-08-2024]
 Notification No.0724/B for various temporary positions on contractual basis - [29-07-2024]
Notification No.0724/B for various temporary positions on contractual basis - [29-07-2024]
 List of shortlisted candidates for the temporary positions against CCMB Web Notif.No.0624/A- [19-07-2024]
List of shortlisted candidates for the temporary positions against CCMB Web Notif.No.0624/A- [19-07-2024]
 List of Provisionally empanelled candidates for Engagement as Project staff Vide Notif.No.2024/1 - [15-07-2024]
List of Provisionally empanelled candidates for Engagement as Project staff Vide Notif.No.2024/1 - [15-07-2024]